
Multilingual FDA Documentation Made Easy
The client faced significant challenges in preparing documentation for a multilingual FDA submission. Their working documentation was multilingual, containing Japanese, Korean, Chinese, and English content.
Specialized translations ensuring compliance with FDA, EMA, and global standards.











At Lingrowth, we specialize in providing precise and culturally adapted translations for the medical and healthcare sectors. With a focus on compliance and innovation, we ensure your content meets the highest standards for global success.
Ensuring precision in protocols, investigator brochures, informed consent forms, and study reports.
Accurate translations for drug registration, packaging, labeling, and patient information leaflets.
Translations tailored to meet technical and regulatory standards for medical device manufacturers.
We are an international language service provider specialized in life science and medical translation services. The quality of the services provided is our priority. The current workload is performed by our team in the following specializations:
63% of translation errors have potential clinical consequences. Our translators, proofreaders and subject matter experts are medical doctors who have medical experience in a particular area and in medical translation services. They can deeply understand and appropriately translate all terms and professional wordings without mistakes.
Questionnaire understanding increases to 98.9% after LV. A widespread network of doctors, LV specialists and Project Managers in the staff of our company allows us to perform this process promptly, qualitatively and with needed results.
Bad UI decreases medtech solutions usability by 43%. We work closely with developers teams using the latest technologies in the localization process and testing the received solution thoroughly.
Lingrowth translation company currently provides medical translation services on the main Western European languages (English, German, French, Spanish, Italian) and Eastern European languages (Russian, Belarusian, Ukrainian, Polish, Czech, Serbian) and some Asian languages (Chinese, Japanese, Korean)
We translate the documents in the following subject matter areas: medicine, pharmaceutical, biotechnology, including patents, regulatory documents, clinical trial data,
Our support includes editing which is performed by other medical linguist who is specialized in the particular subject matter.
For your convenience, we additionally offer proofreading by a professional linguist who is a native speaker of the target language in order to check quality and style according to ISO 17100 international standard.

The client faced significant challenges in preparing documentation for a multilingual FDA submission. Their working documentation was multilingual, containing Japanese, Korean, Chinese, and English content.

Our client is a MLLSP operating in the Europe. Their core requirement is translating medical documentation across European languages. However, a significant challenge they faced was the need for direct translations between less common language pairs without using English as an intermediary
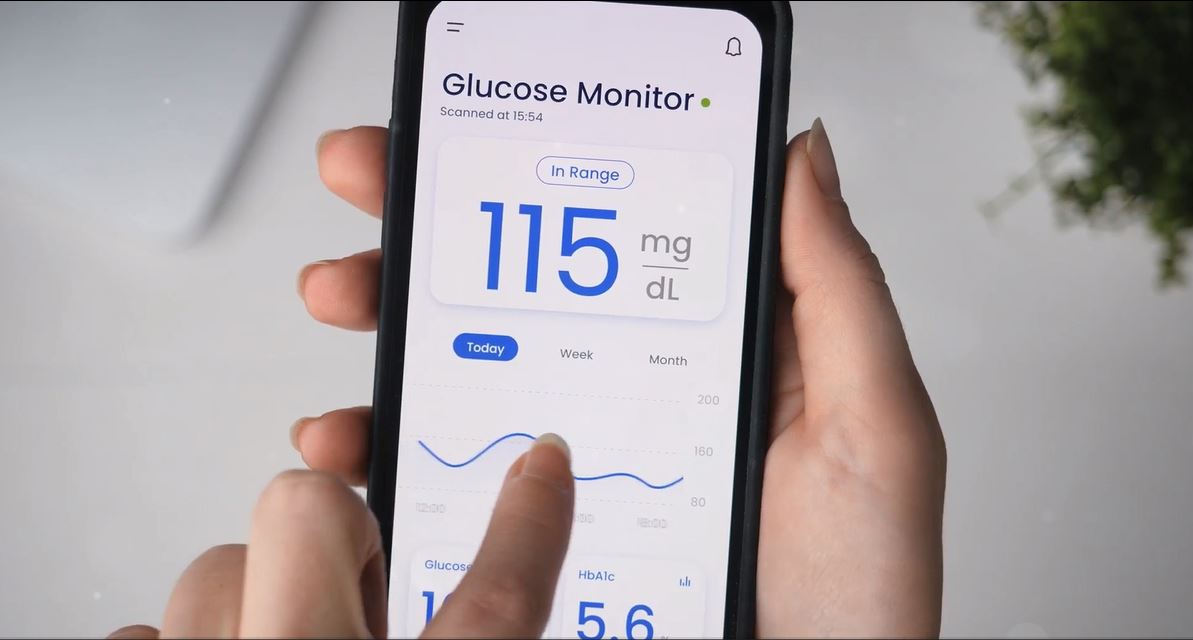
Our client is the global company specialized in medical technology and other healthcare solutions. It was founded in Ireland and operates in different corners of the world now. They innovate, advance, and implement various medical technology to make everyday life easier. The client requested a Medical App Localization service
PROJECTS ANNUALLY
HAPPY CLIENTS
COUNTRIES
PROFESSIONALS
We use cookies and similar technologies to enhance your experience. With your consent, we may process data like browsing behavior or unique IDs. Without consent, some features may not work properly.